Comparative study of antitumor effect Fe2O3 and Fe3O4 nanoparticles in nanocomplex with doxorubicin during electromagnetic irradiation and modification of human hepatocellular carcinoma cells (HepG2) with interferon-alpha
Schepotin I.B.1, Kudrjashov A.G. 2, Skachkova O.V.1, Khranovska N.M., Romanov A.V.1, Nikolov М.О.1
Summary. Conducted experimental studies in human hepatocellular carcinoma cell line HepG2 showed that highest cytotoxic effect was observed when nanocomplex (NC) which consisted of Fe2O3 nanoparticles (NP) with doxorubicin (DR) was used, as well as Fe3O4 NP or a mixture Fe2O3+Fe3O4 with DR after prolonged exposure to interferon-alpha (IFN). The number of apoptotic cells increased when NC which consisted of Fe3O4 and/or Fe2O3 NP with DR was used after electromagnetic irradiation (EI) and prolonged exposure to IFN compared to the experiments when EI was not used. The number of topoisomerase II alpha-positive cells decreased when NC with Fe3O4 NP and DR was used after EI and long-term exposure to IFN compared with experiments when Fe2O3 NP and DR were used.

Introduction
Significant efforts of researchers in oncology is currently focusing on developing magnetosensitive nanocomplexes (NC) based on the synthesis of iron oxide nanoparticles (NP) with cytostatic drugs. NC have additional advantages over traditional drugs because of its ability to integrate with a variety of substances due to the high specific surface area [1]. NC synthesized from low magnetite (Fe3O4) or hematite (γ-Fe2O3) with antineoplastic antibiotic doxorubicin (DR) can be used as anticancer agents in radio frequency hyperthermia [2].
It is known that hematite Fe2O3 is the most stable oxygen compounds of iron, conversely magnetite Fe3O4 (FeO · Fe2O3) is more easily oxidized and it is a good conductor of current and has a large amount of redox standard electrode potential [3]. NC iron oxides include ions with unpaired electrons on the inner membrane (e.g. 3 shell of Fe ions in Fe3O4), which determine the magnetic properties of magnetite. Fe ion has a lower number of unpaired electrons (four) than the ion Fe, for which this value is five [4]. In the process of NC formation which includes DR, there are conditions for transitions between internal and partially filled outer orbitals, which leads to a change in the reactivity of NC [5].
Antitumor activity of NC under the influence of electromagnetic irradiation (EI) is associated with an increase in the ability of anthracyclines in the presence of iron oxide cells metabolized with the formation of free radicals. Quinone part of DR tetracycline ring becomes a free radical semiquinone involving mitochondrial, nuclear and microsomal NADP-oxidoreductases of cells [6]. This initiates apoptosis and necrosis of tumor cells.
Interferon (IFN) in nowadays is one of the well known cytokines, which are used in biotherapy of cancer patients because it has pluripotential effects on tumor cells, such as modifier of phenotype and may increase the sensitivity of tumor cells to anticancer chemotherapy [7, 8]. In experiments on cell lines of colon cancer SW480, COLO and WiDr also shown that the antiproliferative effect of IFN enhanced iron chelate [9]. Based on the above it can be assumed that the antitumor effect of NC DR at EI and modification of cell by IFN probably can depend on the type of compounds of iron and oxygen in NC and different action on tumor cells.
The aim of the work was comparative study of the features of antitumor effect of Fe3O4 and Fe2O3 NP in NC with DR on cell line HepG2 human hepatocarcinomas at EI under their long-term (30 days) exposure to IFN-alpha.
Materials and Methods
Cell culture and exposition with IFN. Human hepatocellular carcinoma cells (HepG2 line) obtained from the Collection of Cell Lines of R.E. Kavetsky Institute of Experimental Pathology, Oncology and Radiobiology (Kyiv, Ukraine) were cultured in complete DMEM culture medium supplemented with 10% embryonal calf serum and 40 µg/ml gentamycine. The cells were cultivated in humidified 5% СО2 atmosphere at 37 °С. Recombinant IFN-alpha-2b («BioPharma», Ukraine) was used for cells modification with cytokine. The cells were cultivated for 30 days in increasing concentration of cytokine (from 500 to 10 000 U/ml). The range of doses was chosen after determination of IC 50 (50% inhibition concentration).
Magnetosensitive NC. As an independent parts or components of drugs NP of iron oxide Fe2O3 and Fe3O4 with diameter<50 nm («Sigma») and DR («Pfizer Italy SRL», Italy) were used. Mass concentration of DR in NC was 50%. NC received by the technology of mechano-magneto-chemical synthesis [10].
EI. The prototype of apparatus «Magniterm» («Radmir», Ukraine) was used for spatially inhomogeneous EI. EI options: frequency was 40 MHz, output power was 60 W, applicator was the framework with dimensions of 2×2.5 cm and profile in the form of an arc with curvature radius of 2.3 cm.
Cytotoxicity. To determine the drugs sensitivity the standard method with vital dyes was used [11]. To assess the effect of combination of EI and investigated drugs cells were grown in Petri dishes (diameter was 40 mm, 2×10 cells/dish) under standard conditions for 24 hours, then added drugs and were irradiated for 30 min.
The number of living and dead cells was determined with haemocytometer after 48 hours of irradiation, stained by trypan blue.
Cytofluorimetry. Flow cytometry was conducted to analyze the influence of DR and EI on the induction of apoptosis of tumor cells and the distribution of cell cycle phases. Flow cytometry was performed on a device FACalibur («Bekton Dickinson», USA) equipped with two lasers: argon and helium-neon with wavelengths of 488 and 625 nm, respectively. The analysis was performed using CellQuest. To measure the fluorescence of propidium iodide was used filter with maximum transmission at 600 nm and a bandwidth of 35 nm. Using flow cytometry the percentage of cells that fall hypodiploidic area of DNA index histogram was assessed.
Statistics. Statistical processing of the data was performed using the Student t-test by the Statistica 6.0 (© StatSoft, Inc.) software with preliminary verification of the hypothesis of normal distribution on the Kolmogorov — Smirnov criterion.
Results and Discussion
Cytotoxicity. Comparison of direct antitumor effects of Fe2O3 and Fe3O4 NP in NC with DR at EI on tumor cells under long-term exposition with IFN had been conducted on HepG2 cell line (Fig. 1). Analysis of the data shows that the highest antitumor effect was observed in NC with DR combination, which included Fe2O3 without EI and IFN (27% living cells) and in a case with Fe3O4 (26%) or a compound of Fe2O3+Fe3O4 (28%) using IFN without EI. In all experiments, the influence of NC with DR on the viability of HepG2 cells was statistically significant differed as compared to control. The NC effect on cell viability depended on the type of oxygen-containing compounds.
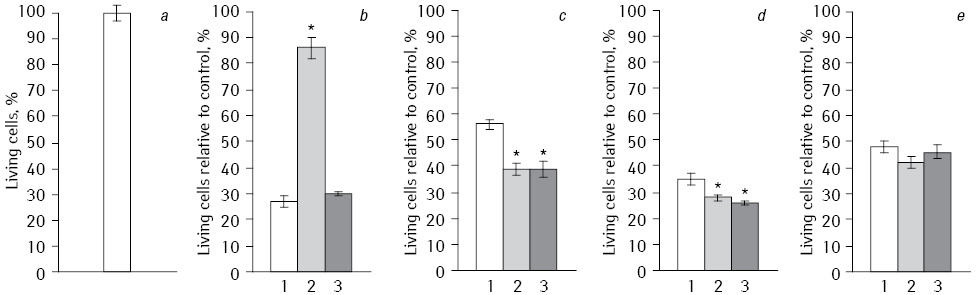
*Statistically significant differences compared to Fe2O3(p<0.05).
Cytometry. For a more detailed understanding of the sensitivity of tumor cells to the drug we investigated an influence of EI, IFN and their combination on the presence of apoptotic cells and the effects of the division of the cell cycle phases by flow cytometry.
Analysis of the apoptosis induction level of HepG2 cells performed by flow cytometry (Fig. 2). When we used NC with Fe3O4 the number of apoptotic cells was minimal, but showed a significant increase in the number of apoptotic cells after exposure with IFN. Thus, the number hypodiploid cells after exposure to NC with Fe3O4+DR was only 3.84%, and in IFN-modified subline increased to 61.62%. During EI and the action of NC with Fe3O4 the smallest number of hypodiploid cells (28.4%) was observed, but after exposure to EI and long-term acting IFN their number increased to 61.49%. In combination EI with NC, which included Fe2O3 with DR, and after prolonged cells exposure with IFN the number of apoptotic cells was significantly increased compared to the experiments without irradiation. In control HepG2 cells number of apoptotic cells did not exceed 10.99%.
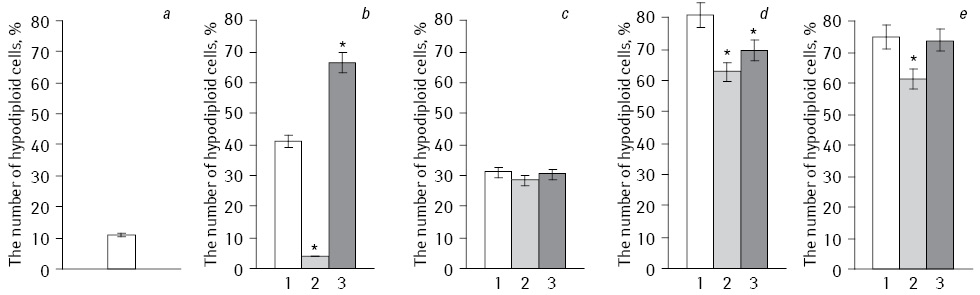
*Statistically significant differences compared to Fe2O3 (p<0.05).
Thus, Fe2O3 and Fe3O4 with EI under modified IFN initiated significant difference in the level of apoptotic cells.
Percentage division of HepG2 cells according to the phases of the cell cycle (G0/G1, S and G2/M) are presented in Fig. 3. Generalized comparative analysis of all conducted experiments concerned to the difference in the action of Fe2O3+DR, Fe3O4+DR, and Fe2O3+Fe3O4+DR on cell cycle phases division is shown in Table 1. Analysis of the data shows that the experiments with NC which included Fe3O4 the average number of cells in S-phase decreased to 26.3% and increased to 38.8% in G0/G1 phase, and to 35% in G2/M phase. In experiments with NP Fe2O3 similar indexes have corresponding values of 35.1, 32.3 and 31.9%. When we used NC Fe2O3+Fe3O4 studied parameters were in intermediate values.
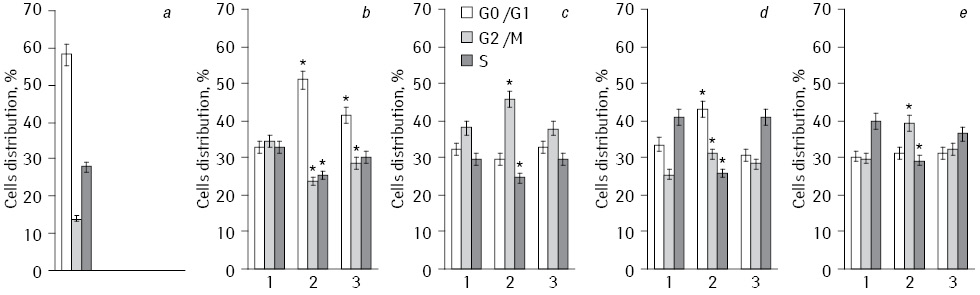
*Statistically significant differences compared to Fe2O3 (p<0.05).
Table 1. Generalized comparative analysis from all conducted experiments of differences in the influence of NP Fe2O3+Fe3O4+DR with DR and Fe2O3+Fe3O4+DR to the changes in the distribution on the phases of the cell cycle HepG2 cell lines (M,%)
| NP | Cell cycle phases | ||
|---|---|---|---|
| G0/G1 | G2/М | S | |
| Fe2O3+DR | 32.3 | 31.9 | 35.1 |
| Fe3O4+DR | 38.8 | 35.0 | 26.3 |
| Fe2O3+Fe3O4+DR | 34.1 | 31.7 | 34.1 |
So, NC of Fe3O4 has decreased percentage distribution cells in S-phase of the cell cycle as compared to Fe2O3 that slowed DNA synthesis. Since the main reactant in the drug is DR, based on prior studies [12, 13] it can be suggested an increase in initiation of cyclic redox reaction of quinones in semyquinones due to enhanced oxidative stress NC of Fe3O4 and DR. Under the action of EI this led to the formation of reactive oxygen, such as anion radical, hydrogen peroxide and hydroxyl radical. These compounds caused DNA damage and slowing cell proliferation.
As to mechanism of prolonged exposure of cells with IFN such differences are the most probably related to known features mediated modulation of iron oxides antiproliferative effects of IFN [14, 15].
For more detailed analysis of NC+EI combinations on tumor cells with additional IFN modification was investigated topoisomerase II alpha (Topo II) — isomerase enzyme that participates in topological transitions of DNA double-stranded breaks by making stabilization complex DNA enzyme. Topo II activity in the cell is known to depend on the phase of the cell cycle. So, an efficacy of topoisomerase inhibitors correlates with the degree of proliferative activity of cells, particularly the portion of cells in S-phase. Blocking the cell cycle in G1-phase causes resistance to drugs Topo II inhibitors [16]. For example, Topo II inhibitors — etoposide stabilizes isomerase and prevents repair of DNA break, which is a signal to start the process of apoptosis [17]. After that p53, p21WAF proteins activated, cytochrome C released, occurs activation of caspase 8, 9, 3 and induction of cell cycle blocking. In addition, there is evidence that the expression of Topo IIa also associated with expression of Ki-67 which is a marker of cell proliferation. Topo II is involved in proliferation, differentiation and cell sensitivity to anticancer agents. Topo II activity reflects signal changes at molecular level in tumor cells [18].
We found a significant reduction of Topo II-positive cells after prolonged exposure to IFN and after the action on cells EI in combination with Fe3O4+DR compared to Fe2O3+DR (Table 2). This is especially evident in the analysis of cells with the cytoplasmic localization of this enzyme (Fig. 4, 5). In addition, we observed a significant inhibition of Topo II in the nucleus of cells in the control subline after Fe2O3+DR influence, which correlates with the fewest number of living cells in cytotoxicity test.
Table 2. The effect of combination NC with DR and EI after long-term incubation with IFN on the number of Topo II-positive cells
| N | Preparation | Positive cells (by Н-score system), estimation in points | |||
|---|---|---|---|---|---|
| Control cells | Modified by IFN | ||||
| Nuclei | Cytoplasm | Nuclei | Cytoplasm | ||
| 1 | Control | 38±5.5 | 25±8.5 | <10 | 22±4.0 |
| 2 | Fe2O3+DR | <10 | 54±11.0 | 84±10.0 | 71±9.0 |
| 3 | Fe3O4+DR | 50±8.0 | 79±4.5 | 37±1.0 | 74±8.5 |
| 4 | Fe2O3+Fe3O4+DR | 57±4.5 | 59±7.0 | 39±4.5 | 31±7.5 |
| 5 | Fe2O3+DR+EI | <10 | 27±3.0 | 32±5.0 | 40±0.0 |
| 6 | Fe3O4+DR+EI | 38±3.0 | 24±2.5 | 21±2.0 | 11±3.0 |
| 7 | Fe2O3+Fe3O4+DR+EI | 61±7.0 | 19±3.0 | 22±3.5 | 21±1.0 |
Statistically significant difference to Fe2O3+DR+EI (p<0.05).
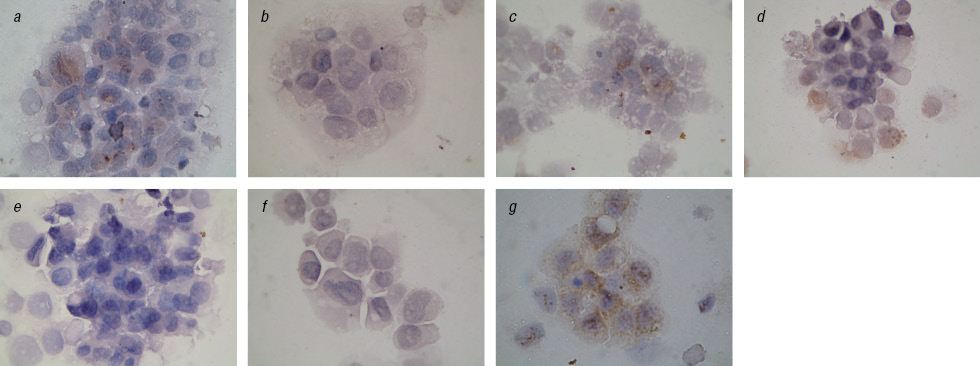
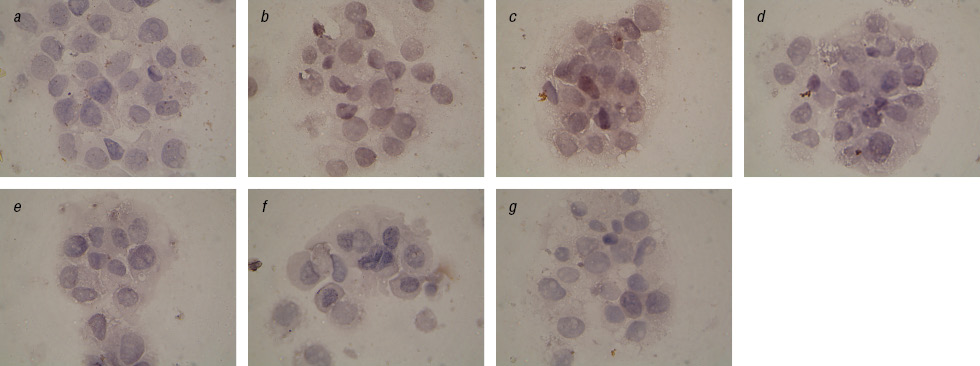
These facts are interesting because are known clinical observations on the acquisition of cell resistance to anticancer drugs with increasing Topo II expression in the cytoplasm and the worst prognosis on survival of patients with an increase expression of Topo II in the tumor cells nucleus [19, 20]. According to our data decrease in the Topo II expression occurs after long-term exposition with IFN particularly in the case of specific complexes. This may reflect the increasing sensitivity of tumor cells to chemotherapy and therefore inhibit Topo II enzyme, and increase the percentage of apoptotic cells [21].
Thus, summarizing the results we could confirm that the antitumor effect of NC, which includes iron oxide + DR with EI and IFN-modification depends on the type of compounds of iron and oxygen and more expressive when using compound Fe3O4 with DR. We can assume that the dependence of the antitumor effect of NC-type compound oxide of DR was conditioned by the phenomenon of spin-dependent electron transport between iron oxide and aromatic rings DR during EI, resulting in the benzene ring DR activated different levels of magnetic ring current whose magnitude modulated level of oxidative stress in malignant tumors.
Conclusions
1. The greatest cytotoxic effect on HepG2 cell line was found using NC which included Fe2O3 with DR without EI and in complex of Fe3O4 or Fe2O3+Fe3O4 with DR after prolonged exposure of cells with IFN-alpha.
2. During EI of NC, which included NP Fe3O4 and/or Fe2O3 with DR, after prolonged exposure of cells with IFN-alpha the number of apoptotic cells HepG2 is more significantly increased compared to experiments without using electromagnetic radiation.
3. During EI of NC, which included Fe3O4 NP with DR Topo II alpha-positive cells HepG2 was decreased as compared to Fe2O3 NP after their prolonged exposure to IFN-alpha.
References
1. Roca A.G., Costa R., Rebolledo A.F. et al. (2009) Progress in preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D. Appl. Phys., 42: 1–11.
2. Nedelcu G. (2008) Magnetic nanoparticles impact on tumoral cells in the treatment by magnetic fluid hyperthermia. Digest J. Nanomat. Biostruct., 3(3): 103–107.
3. Harris L.A. (2002) Polymer Stabilized Magnetite Nanoparticles and Poly (propylene oxide) Modified Styrene-Dimethacrylate Networks. Dissertation doctor of philosophy in chemistry. Blacksburg: Virginia, 161 р.
4. Figgis B.N., Lewis J. (1960) The Magnetochemistry of Complex Compounds. In: J. Lewis and R.G. Wilkins., Modern Coordination Chemistry. New York: Wiley, 524 p.
5. Symons M., Gutteridge J. (1998) Free Radicals and Iron: Chemistry, Biology, and Medicine. Oxford University Press, 242 p.
6. Орел В.Э., Щепотин И.Б., Смоланка И.И. и др. (2012) Радиочастотная гипертермия злокачественных новообразований, нанотехнологии и динамический хаос. ТПМУ Укрмедкнига, Тернополь, 448 c.
7. Кудрявець Ю.Й., Бездєнєжних Н.О., Лихова О.О. та ін. (2009) Механізми модифікації чутливості клітин раку легені людини до протипухлинних хіміопрепаратів за умов тривалої експозиції клітин з альфа-інтерфероном. Вісник наукових досліджень, 57(4): 102–104.
8. Kudryavets Yu.I., Bezdenezhnykh N.O., Lukyanova N.Yu. et al. (2008) Modifying influence of prolonged action of interferon on phenotypic characteristics of human lung cancer cells in vitro. Exp. Oncol., 30(4): 283–288.
9. Mori S., Sawada T., Okada T., Kubota K. (2008) Anti-proliferative effect of interferon-gamma is enhanced by iron chelation in colon cancer cell lines in vitro. Hepatogastroenterology, 55(85): 1274–1279.
10. Орел В.Е., Шевченко А.Д. Мельник Ю.Г. и др. (2010) Физико-химические характеристики магниточувствительного нанокомплекса, полученного с использованием механомагнетохимической технологии сухого синтеза. Металлофизика и новейшие технологии, 32(9): 1157–1167.
11. Орел В.Е., Бездєнєжних Н.О., Шевченко А.Д. та ін. (2011) Дослідження впливу електромагнітного опромінення та магніточутливого нанокомплексу на клітини аденокарциноми. Клиническая онкология, 4(4): 148–152.
12. Minotti G., Menna P., Salvetorelli E., Cairo G., Gianni L. (2004) Anthracyclines: molecular advances and pharmacological developments in antitumor activity and cardiotoxicity. Pharmacol. Rev., 56: 185–229.
13. Shapira M., Segal E., Botstein D. (2004) Disruption of yeast forkhead-associated cell cycle transcription by oxidative stress. Mol. Biol. Cell., 15: 5659–69.
14. Weiss G., Fuchs D., Hausen A. et al. (1992) Iron modulates interferon-gamma effects in the human myelomonocytic cell line THP-1. Exp. Hematol., 20(5): 605–610.
15. Regis G., Bosticardo M., Conti L. et al. (2005) Iron regulates T-lymphocyte sensitivity to the IFN-gamma/STAT1 signaling pathway in vitro and in vivo. Blood, 105(8): 3214–21.
16. Свирновский А.И., Григорович С.А. (2005) Плейотропная резистентность опухолевых клеток к терапевтическим воздействиям при В-клеточных лимфопролиферативных заболеваниях. Медицинские новости, 9: 5–16.
17. Chen Q., Gong B., Almasan A. (2000) Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis .Cell Death Differ., 7(2): 227–233.
18. Liu D., Huang C.-L., Kameyama K. et al. (2002) Topoisomerase IIα gene expression is regulated by the p53 tumor suppressor gene in nonsmall cell lung carcinoma patients. Cancer, 94(8): 2239–47.
19. Watanuki A., Ohwada S., Fukusato T. et al. (2002) Prognostic significance of DNA topoisomerase IIalpha expression in human hepatocellular carcinoma. Anticancer Research, 22(2B): 1113–1119.
20. Chen M.C., Chen C.H., Chuang H.C. et al. (2011) Novel mechanism by which histone deacetylase inhibitors facilitate topoisomerase IIα degradation in hepatocellular carcinoma cells. Hepatology, 53(1): 148–159.
21. Ozgur O., Karti S., Sonmez M. et al. (2003) Effects of interferon-alpha-2a on human hepatoma HepG2 cells. Exp. Oncol., 25: 105–107.














Leave a comment