Analysis of complications after radical treatment of rectal cancer. VAC therapy as a new method of problem solving
Partykevych Y.D.1, Maliborska S.V.2
- 1Ivano-Frankivsk National Medical University, Ukraine
- 2Precarpathian Clinical Oncology Centеr, Ivano-Frankivsk, Ukraine
Summary. Aim. To increase the effectiveness of treatment of anastomosis leakage in patients with rectal cancer after neoadjuvant therapy. Materials and methods. We searched and analyzed publications in PubMED for 2015–2022, which provided data on the use of negative pressure endovacuum therapy in patients with anastomotic leakage after surgical treatment. The search queries related to the tags «rectal cancer», «postoperative complications», «anastomotic leakage», «neoadjuvant therapy», «negative pressure vacuum therapy», «endovacuum therapy». The results of treatment of patients with stage II–III rectal adenocarcinoma (T2-3N0-2M0) who underwent surgical treatment at the Precarpathian Clinical Oncology Center of the Ivano-Frankivsk Regional Council in 2019–2022 were systematized and analyzed. The study included 97 patients with rectal cancer and no other oncological diseases. All patients received combined treatment according to international National Comprehensive Cancer Network (NCCN) guidelines. At the first stage, patients underwent preoperative telegamma therapy to TFD of 25–40 Gy, followed by restaging with magnetic resonance imaging (MRI) and surgical treatment in a standard volume with high ligation of the inferior mesenteric artery and compliance with total mesorectumectomy (TME) principles. All patients underwent a preventive colostomy (ileo/transverse) and received perioperative or adjuvant chemotherapy as needed. Anastomotic leakage was diagnosed clinically, in the laboratory (C-reactive protein, procalcitonin), using a Fujinon EC 201 WL video colonoscope or a rectoscope, proctography, and pelvic MRI using a Magnetom Espree (Siemens) with a magnetic field strength of 1.5. Leakage assessment was graded according to the Clavien-Dindo scale and the leakage assessment score. Results. Traditional treatment of anastomotic leakage can last up to 1 year, and in 40–60% of cases satisfactory results are not achieved, which makes colostomy reversal impossible. On the basis of the Precarpathian Clinical Oncology Center of the Ivano-Frankivsk Regional Council, the traditional treatment is applied with the use of antibiotic therapy and topical treatment; the duration of healing of the anastomotic defect is on average 4–6 months (120–180 days), and in 30–40% of cases, colostomy reversal is not achieved. The endovacuum aspiration therapy system is a new method that, even with significant defects, allows to achieve satisfactory results and improves the quality of life of patients. When using endoscopic vacuum-assisted closure (EVAC) therapy, on average, 4–5 polyurethane sponges are used and 4–5 sessions of vacuum therapy are performed with an average pressure of 70–120 mmHg, with the time for sponge replacement varying from 3–6 days. The average treatment duration is 30–60 days. Colostomy reversal is achieved in 60–70% of patients. When using stationary vacuum assisted closure (VAC) therapy systems, patients stay in the hospital for up to 30–50 days; while using new portable systems, such as Renesys Go, it is possible to treat this category of patients on an outpatient basis. Conclusions. Anastomotic leakage remains an urgent problem today. Neoadjuvant treatment of patients with rectal cancer has made significant progress in controlling the disease and prolonging the recurrence-free life expectancy. However, surgical treatment in compliance with the TME principles remains the gold standard. The early diagnosis and treatment of this type of anastomosis leakage not studied. Standard methods of treatment do not give satisfactory results. After all, the duration of treatment can be up to 1 year or more. And in many cases, the colostomy reversal cannot be achieved, which contributes to the lifelong disability of patients. The use of endoVAC therapy can become a new and effective way of treating anastomotic leakage and presacral abscesses with a success rate of up to 70%. Based on our study, the optimal pressure is 70–120 mmHg, with sponge replacement every 4–5 days, depending on the amount of exudate, thus it is possible to achieve granulation tissue, which contributes to reducing the speed of healing of the defect.
Received 12.11.2023
Accepted for publication 08.12.2023
DOI: 10.32471/clinicaloncology.2663-466X.52-4.31352
INTRODUCTION
Colorectal cancer remains the third leading cause of death among all cancers [1, 2]. The introduction of the concept of combined treatment using perioperative chemotherapy and telegamma therapy has significantly increased the survival rate of patients [3]. However, surgical treatment in compliance with all TME principles and high ligation of the inferior mesenteric artery is still the gold standard [1].
Anastomotic leakage after the surgical stage of treatment can occur in 0.0 to 36.3% of cases after TME [4]. Anastomotic leakage is a serious complication for patients and requires appropriate treatment.
The process of anastomosis healing is a complex, multifactorial process that goes through various stages and is primarily determined by the total amount of collagen deposition at the anastomosis site, as well as the transverse suturing of its fibers. The rate of anastomosis healing is influenced by various factors, which are divided into systemic and local ones. Systemic factors include age, nutritional status, comorbidities, and neoadjuvant chemotherapy. While the local factors include anastomotic blood supply disorders, surgical technique violations, and preoperative telegamma therapy [5]. Anastomotic leakage remains the most threatening complication of conventional and laparoscopic colorectal surgery today, which increases the duration of hospitalization, mortality and risk of reoperation, decreases the quality of life and the length of time colostomy reversal, and sometimes disables patients [6]. Whereas subclinical recognition of anastomotic leakage (class A-B) is based mainly on non-objective criteria, so this type of complication is often diagnosed only after control examinations, in particular proctography and MRI. The incidence of anastomotic leakages varies significantly, ranging from 1 to 24%, as reported in different studies. In large colorectal centers, the incidence of leakage ranges from 3.4–5.3% [7, 8]. Thus, the issue of treating this complication using various methods has become urgent, and although a few years ago the «watch and wait» option for small defects (up to 2 cm) was the main option, today a minimally invasive method of treatment using a complex of EVAC is increasingly being developed, which has helped to reduce the duration of anastomotic healing and the time to reverse the transversostomy [9].
MATERIALS AND METHODS
The research program was approved by the Bioethics Commission of the Ivano-Frankivsk National Medical University.
We searched and analyzed publications in PubMED for 2015–2022, which provided data on the use of negative pressure endovacuum therapy in patients with anastomotic leakage after surgical treatment. The search queries related to the tags «rectal cancer», «postoperative complications», «anastomotic leakage», «neoadjuvant therapy», «negative pressure vacuum therapy», «endovacuum therapy».
The results of treatment of patients with stage II–III rectal adenocarcinoma (T2-3N0-2M0) who underwent surgical treatment at the Precarpathian Clinical Oncology Center of the Ivano-Frankivsk Regional Council in 2019–2022 were systematized and analyzed. The study included 97 patients with rectal cancer and no other oncological diseases. All patients received combined treatment according to international NCCN guidelines. At the first stage, patients underwent preoperative telegamma therapy to TFD of 25–40 Gy, followed by restaging with MRI and surgical treatment in a standard volume with high ligation of the inferior mesenteric artery and compliance with TME principles [1]. All patients underwent a preventive colostomy (ileo/transverse) and received perioperative or adjuvant chemotherapy as needed. Anastomotic leakage was diagnosed clinically, in the laboratory (C-reactive protein, procalcitonin), using a Fujinon EC 201 WL video colonoscope or a rectoscope, proctography, and pelvic MRI using a Magnetom Espree (Siemens) with a magnetic field strength of 1.5. Leakage assessment was graded according to the Clavien-Dindo scale and the leakage assessment score. All patients gave their consent to research and processing of their personal data.
RESULTS OF THE STUDY
A large Dutch study found that stoma reversal could not be achieved in 49% of patients with anastomotic leakage [10]. Thus, there is an urgent need to study various options for treating anastomotic leakage in patients without peritonitis who underwent low anterior resection with a preventive ileo/transverse stoma.
Since the first data on wound treatment with vacuum therapy appeared in the late 1990s, this technology has covered almost all areas of surgery, providing a versatile and easy-to-use method of treating complex wounds [11].
In 2001, Weidenhagen et al. began to implement endoscopic vacuum therapy for the treatment of colorectal anastomotic leakage as a minimally invasive method of continuous and effective drainage of perianastomotic abscesses and fistulas [12].
Despite the fact that studies in the treatment of anastomotic leakage in colorectal cancer using endoscopic vacuum therapy are characterized by high success, there is a lack of data on the advantages of different methods of this type of treatment [13, 14].
In 2007, an international group for the study of rectal cancer tried to standardize this method of treatment. Types of various techniques differed depending on the location of the anastomosis, the size of the presacral abscess or the size of the defect, and the patient’s clinical presentation. Nevertheless, if there is a large defect (more than 180 degrees), even for a clinically stable patient, the use of endoVAC therapy is not recommended [15, 16].
Endoscopic treatment of anastomotic leakage is based on the transanal placement of a microporous sponge directly in the anastomotic site or in the perianastomotic cavity (abscess cavity), which is connected to the mechanism that creates a constant negative pressure in the cavity. The sponge is usually changed every 2–4 days. Active drainage with constant negative pressure contributes to the reduction of the defect and leads to a decrease in local edema, which improves perfusion and promotes the rapid formation of granulation tissue [12].
Thus, endoscopic vacuum therapy has become the method of choice in the treatment of anastomotic leakage after rectal resection. Florian and other researchers reported that there was no difference in mortality when treating this complication (p=0.624) [17]. However, the success rate of treatment with endoscopic VAC therapy was significantly higher compared to standard treatment methods (antibiotic therapy, anti-inflammatory and topical therapy) (95.2 vs. 65.8%). The degree of anastomtic healing with the help of endoVAC therapy ranges from 56–97%. 70% of patients in this study received preoperative telegamma therapy [17].
The duration of healing of anastomotic leakage in one of the studies was 6 months in 16/30 patients (53%), while anastomotic healing within 6 months in those who did not receive neoadjuvant therapy was observed in 7 out of 8 patients (more than 90%). The anastomosis was considered intact if there were no signs of contrast extravasation during MRI or proctography.
Another multicenter study showed a success rate of 81.4% for endoVAC therapy, with an average stoma reversal rate of 66.7%. They found that various risk factors can affect the success of endoVAC therapy, such as neoadjuvant chemoradiotherapy [11].
Von Bernstorff et al. first evaluated the effect of radiation therapy on the anastomotic healing process during endoVAC therapy. The aforementioned authors reported a longer treatment duration, a greater number of sponge use, and longer time to closure of the abscess cavity [18–21].
In contrast, other researchers did not find any correlation of neoadjuvant therapy with leakage healing time and success rate [13, 22].
The overall incidence of complications associated with the use of endoVAC therapy has an average value of 11.1%. In particular, the most common complication was recurrence of presacral abscess [11].
Other researchers reported such complication as anastomotic stricture, reaching about 33%, which was eliminated by balloon dilation [13]. In some studies, the occurrence of moderate pain as a complication of endoVAC therapy was observed in 36% [5]. During the sponge replacement and migration into the abdominal cavity, another issue arose—bleeding, with an incidence not exceeding 1% [23].
Another multicenter prospective study found that when the distance from the anal sphincter to the anastomosis was 4.9–10 cm and the size of the abscess was 5–8.1 cm, an average of 7 sponges (2–34) were used with an average negative pressure of 150 mmHg, and the average healing time was 31 days (14–127 days). The success rate was 85.4% [13].
In most studies, the endoscopic vacuum device consisted of a 7×3 cm open-cell polyurethane sponge; the sponge was completely inserted into the abscess cavity using an introducer system. The end of the probe was connected to various negative pressure vacuum systems. A constant negative pressure of 120–150 mmHg was created [24].
Thus, anastomotic leakage is one of the most dangerous complications after surgical treatment of rectal cancer with TME [25].
The minimal invasiveness of endoVAC therapy is associated with low costs and low risk from the procedure itself [26]. Depending on the size of the defect, successful anastomosis can be achieved in as many as 70–80% of cases [27]. Stoma reversal can be achieved in 63% of cases, but some data indicate a disappointing 40% success rate of treatment [13].
However, other data indicate almost 73% success rate of stoma reversal after endoVAC therapy [5].
The main advantage of this procedure is to ensure continuous drainage of the abscess cavity. The results of various studies are promising, as they allow preservation of the anastomosis compared to standard treatments, including endoscopic excision of the defect, stent placement, antibiotic therapy, and percutaneous drainage. Despite the fact that this procedure is gaining acceptance among the surgical community, the inclusion criteria and determination of effectiveness are not yet standardized and are extremely heterogeneous.
Some researchers report a success rate of endoscopic vacuum therapy from 60 to 100% and a stoma reversal rate of 31–100% [13]. The lack of standardization in the treatment of endoVAC therapy promotes further research in this area.
In a critical analysis, Andrea Vignali and Paola De Nardi prove that VAC therapy in clinically stable patients without peritonitis represents a valid alternative to the conservative approach (stoma diversion, drainage) with a relatively low rate of complications, a higher rate of stoma closure and a shorter hospital stay [28].
Leif Schiffmann, Nicole Wedermann, Frank Schwandner, Michael Gock, Ernst Klar and Florian Kühn evaluated the effect of neoadjuvant chemoradiotherapy on the healing process of anastomotic leakage during VAC therapy. According to the results of their study, there was no difference in mortality (0%), success rate (90.9% vs. 100%), or stoma closure rate (63.6% vs. 62.5%).
After neoadjuvant radiochemotherapy, patients had a significantly longer duration of VAC therapy (31.1 days vs. 15.9 days), which was associated with a higher number of sponge replacements (9.6 vs. 5.0) [29].
K. Talboom et al. in a retrospective cohort study proved that initiation of endoscopic vacuum-assisted surgical closure (EVASC) within 3 weeks is essential for the successful restoration of bowel continuity after anastomotic leakage following rectal cancer resection. EVASC has proven to be progressively successful with the introduction of highly selective diversion and early diagnosis of leaks within 2 weeks, resulting in healing and anastomotic functionality approaching 100% [30].
When analyzing the results of treatment and complications of patients with rectal adenocarcinoma at the Precarpathian Clinical Oncology Center of the Ivano-Frankivsk Regional Council, a total of 97 clinical cases were studied. Complications after surgical treatment were noted in 18.56% (18) of patients according to the Clavien-Dindo scale (Table 1) [31].
| n | % | ±m | |
| Leakage А | 7 | 38.89 | 11.49 |
| Leakage В | 2 | 11.11 | 7.41 |
| Leakage С | 2 | 11.11 | 7.41 |
| Mesentric/ileus | 4 | 22.22 | 9.80 |
| SSI | 2 | 11.11 | 7.41 |
| Eventeration | 1 | 5.56 | 5.40 |
The Clavien-Dindo scale indicates that complications occur in 18.56% of cases. Among these, the most prevalent are Class A leakage at 38.89% and Class B at 11.11%. Consequently, the combined percentage of Class A-B anastomosis leakages within all complications is 9.28%.
We Performed Standard treatment which included antibiotic therapy, transrectal sanitation, abscess opening, and using the endoVAC therapy system.
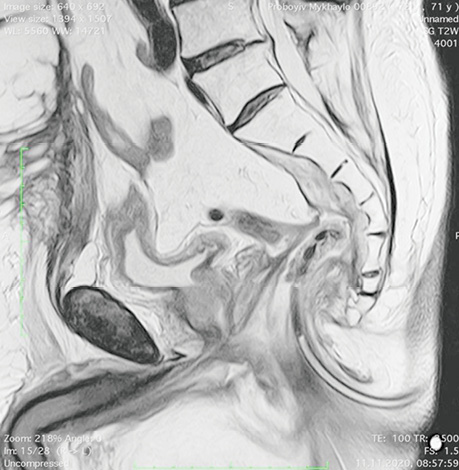
Patient P., born in 1949, with a diagnosis rectal adenocarcinoma (урT3N0M0) underwent combined treatment in the form of preoperative telegamma therapy to TFD 40 Gy, after which a low anterior resection with a preventive transversostomy was carried out. The patient had type II diabetes mellitus, insulin independent and with confirmed angiopathy; the average blood sugar level was 7–9 mmol/l, and grade II obesity. The control examination 2 months after the operation included: MRI, proctography and rectoscopy (Fig. 1), anastomotic leakage and presacral abscess measuring 6×9 cm were diagnosed, according to Clavien-Dindo classification IIIA, according to the leakage score — class B, the patient complained of purulent-fibrinous discharge from the rectum. The size of the anastomosis defect was 2.5–3 cm (Fig. 2).
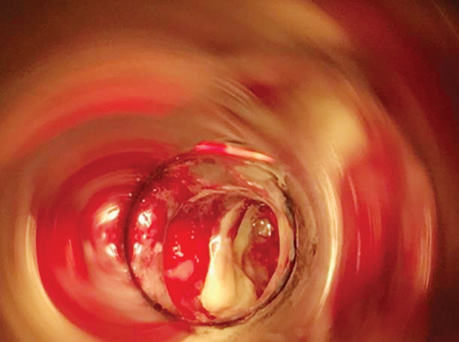
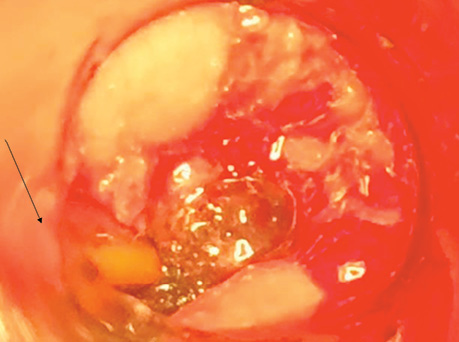
The patient had transanal endoVAC sponge inserted into the paracolostomy abscess. 3 sessions were performed with the sponge changed every 4–5 days. The amount of purulent-fibrinous exudate during this period was 700 ml. A constant negative pressure of 70 mmHg was applied. In the course of endoVAC therapy, MRI monitoring, rectoscopy and proctography were performed 60 days after the diagnosis of anastomotic leakage. Stoma reversal took place 130 days after the initial surgery (Fig. 3).
Description of the device: we used the endoVAC system, which included a polyurethane sponge with a system that was connected to the Renesys Go device with a pressure of 70 mm Hg (Fig. 4).

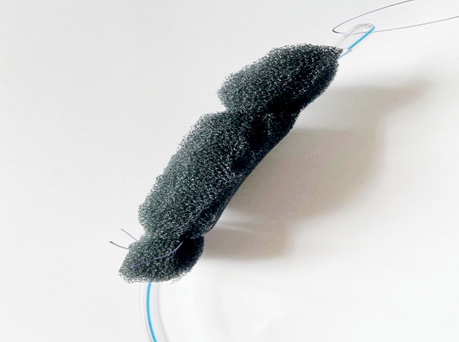
Also, we created an alternative version of the endoVAC sponge (Fig. 5), which included an endopolyurethane sponge that was cut according to the size of the cavity. A cavity up to 0.5 cm in size was made inside the sponge into which a drainage tube with perforated holes was inserted. The sponge was sutured to the tube with Prolene 3.0 thread for fixation, and this system (Fig. 5) was connected to the VAC-device (Renesys Go).
Patient Ya., born in 1956, diagnosed with rectal adenocarcinoma (урТ3N0M0), underwent combined treatment of preoperative telegamma therapy to TFD 40 Gy, after which a low anterior resection with a preventive transversostomy was carried out. 30 days after the operation, the patient complained of fever to 38–39 °C, pelvic pain. MRI, proctography, and rectoscopy diagnosed anastomotic leakage, a presacral abscess measuring 4×5 cm, according to the Clavien-Dindo classification IIIA, class B according to the leakage score. The size of the leakage was 2.5–3 cm. An endoVAC sponge was inserted transrectally into the paracolostomy abscess. There were 2 sessions with a change of sponge every 4–5 days. The amount of purulent-fibrinous exudate during this period was 400 ml. A constant negative pressure of 120 mmHg was applied. MRI control during the course of endoVAC therapy showed a decrease in the leakage up to 1.5–2 cm and the absence of a clinical picture of SIRS. The patient was transferred to the category of «watch and wait» patients for further closure of the transversostomy.
Patient Ya., born in 1959, with a diagnosis of rectal adenocarcinoma (урТ0N0M0). Condition after combined treatment. He received a course of telegamma therapy to TFD 60 Gy. A low anterior resection of the rectum was performed. After 2 months, an anastomotic defect was detected during a follow-up MRI examination. The patient complained of pelvic pain. Anti-inflammatory therapy, antibiotic therapy and topical treatment were performed, but the reversal of the stoma was not achieved.
In the remaining 6 patients, the average time from low anterior resection surgery to follow-up examination, MRI, and CT was 2–3 months (60–90 days). In case of class A-B leakage, traditional antibiotic therapy and anti-inflammatory therapy were performed for 3–4 weeks (21–30 days), followed by another 30–60 days of «watch and wait» tactics. And only after an additional follow-up examination without an anastomotic defect, the colostomy reversal was performed. The average time of treatment and before stoma reversal was up to 1 year (180–360 days).
DISCUSSION AND DEBATE
After searching and analyzing PubMED publications, we found that anastomotic leakage remains a rather complex issue, because despite the development and achievements of modern medicine, the introduction of minimally invasive approaches and the use of Enhanced recovery after surgery (ERAS) protocols, even in the largest colorectal centers of Europe and the United States, reaches 15–40%.
On the basis of the Precarpathian Clinical Oncology Center, the number of complications according to the Clavien-Dindo classification is 18.56%, of which the largest share are class A leakages — 38.89% and class B — 11.11%. Thus, the total number of class A–B anastomotic leakages from all complications is 9.28%. Depending on the severity of the complication, patients are classified according to the Clavien-Dindo scale. Leakages are classified into classes A–C. Whereas class C has symptoms of systemic inflammatory response syndrome (SIRS) and sepsis and patients require immediate surgical treatment, class A–B usually has no obvious symptoms and is diagnosed at later stages, during control examinations or MRI before stoma reversal. This problem remains urgent, because in addition to discomfort, disability (stoma), and economic costs (colostomy bags), patients remain uncertain about further treatment for a long time.
Traditional treatment can last up to 1 year, and 40–60% of patients do not achieve satisfactory results, which makes colostomy reversal impossible. On the basis of the Precarpathian Clinical Oncology Center of the Ivano-Frankivsk Regional Council, the traditional treatment is applied with the use of antibiotic therapy and topical treatment; the duration of healing of the anastomotic defect is on average 6 months — 1 year (180–320 days), and in 30–40% of cases, the colostomy reversal is not achieved.
The vacuum therapy system is a new method that, even with significant defects, allows to achieve satisfactory results and improves the quality of life of patients. When using VAC therapy, on average, 4–5 polyurethane sponges were used and 4–5 sessions of endoVAC therapy were performed with an average pressure of 70–120 mmHg. The duration of treatment is 30–60 days. Colostomy reversal can be achieved in 60–70% of patients. When using standard stationary VAC therapy systems, patients stay in the hospital for a long time, up to 30–50 days; while using new portable systems, such as Renesys Go, it is possible to treat this category of patients on an outpatient basis.
СONCLUSIONS
Anastomotic leakage remains an urgent problem today. The use of endoVAC therapy can become a new and effective way of treating anastomotic leakage and presacral abscesses with a success rate of up to 70%.
REFERENCES
1. Wang, X., Gao, Y., Li, J., Wu, J., & Wang, B. (2018). Diagnostic accuracy of endoscopic ultrasound, computed tomography, magnetic resonance imaging, and endorectal ultrasonography for detecting lymph node involvement in patients with rectal cancer: A protocol for an overview of systematic reviews. Medicine, 97(43), e12899. doi.org/10.1097/MD.0000000000012899.
2. Colorectal Cancer: Statistics (2023). Retrieved from http://www.cancer.net/cancer-types/colorectal-cancer/statistics.
3. Borstlap, W. A. A., Musters, G. D., Stassen, L. P. S., van Westreenen, H. L., Hess, D., van Dieren, S., … Bemelman, W. A. (2018). Vacuum-assisted early transanal closure of leaking low colorectal anastomoses: the CLEAN study. Surgical Endoscopy, 32(1), 315–327. doi.org/10.1007/s00464-017-5679-6.
4. Reisinger, K. W., Poeze, M., Hulsewé, K. W., van Acker, B. A., van Bijnen, A. A., Hoofwijk, A. G., Stoot, J. H., … Derikx, J. P. (2014). Accurate prediction of anastomotic leakage after colorectal surgery using plasma markers for intestinal damage and inflammation. Journal of the American College of Surgeons, 219(4), 744–751. doi.org/10.1016/j.jamcollsurg.2014.06.011.
5. Kühn, F., Janisch, F., Schwandner, F., Gock, M., Wedermann, N., Witte, M., …. Schiffmann, L. (2020). Comparison Between Endoscopic Vacuum Therapy and Conventional Treatment for Leakage After Rectal Resection. World Journal of Surgery, 44(4), 1277–1282. doi.org/10.1007/s00268-019-05349-5.
6. Weber, K., Merkel, S., Perrakis, A., & Hohenberger, W. (2013). Is there a disadvantage to radical lymph node dissection in colon cancer? International Journal of Colorectal Disease, 28(2), 217–226. doi.org/10.1007/s00384-012-1564-x.
7. Wirth, K., Näpflin, M., Graber, S. M., & Blozik, E. (2022). Does hospital volume affect outcomes after abdominal cancer surgery: an analysis of Swiss health insurance claims data. BMC Health Services Research, 22(1), 262. doi.org/10.1186/s12913-022-07513-5.
8. Raptis, D., Pramateftakis, M. G., & Kanellos, I. (2018). Our 20-year experience with experimental colonic anastomotic healing. Journal of Medicine and Life, 11(1), 5–14.
9. den Dulk, M., Smit, M., Peeters, K. C., Kranenbarg, E. M., Rutten, H. J., Wiggers, T., … van de Velde, C.J. (2007). A multivariate analysis of limiting factors for stoma reversal in patients with rectal cancer entered into the total mesorectal excision (TME) trial: a retrospective study. The Lancet. Oncology, 8(4), 297–303. doi.org/10.1016/S1470-2045(07)70047-5.
10. Peinemann, F. (2017). Vakuumversiegelungstherapie von Wunden: randomisierte Studien von 2000 bis 2015 [Negative Pressure Wound Therapy: Randomised Controlled Trials from 2000 to 2015]. Zentralblatt fur Chirurgie, 142(3), 267–274. doi.org/10.1055/s-0043-104697.
11. Weidenhagen, R., Gruetzner, K. U., Wiecken, T., Spelsberg, F., & Jauch, K. W. (2008). Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surgical Endoscopy, 22(8), 1818–1825. doi.org/10.1007/s00464-007-9706-x.
12. Shalaby, M., Emile, S., Elfeki, H., Sakr, A., Wexner, S. D., & Sileri, P. (2018). Systematic review of endoluminal vacuum-assisted therapy as salvage treatment for rectal anastomotic leakage. BJS Open, 3(2), 153–160. doi.org/10.1002/bjs5.50124.
13. Popivanov, G. I., Mutafchiyski, V. M., Cirocchi, R., Chipeva, S. D., Vasilev, V. V., Kjossev, K. T., & Tabakov, M. S. (2020). Endoluminal negative pressure therapy in colorectal anastomotic leaks. Colorectal Disease, 22(3), 243–253. doi.org/10.1111/codi.14754.
14. Blumetti, J., Chaudhry, V., Cintron, J. R., Park, J. J., Marecik, S., Harrison, J. L., … Abcarian, H. (2014). Management of anastomotic leak: lessons learned from a large colon and rectal surgery training program. World Journal of Surgery, 38(4), 985–991. doi.org/10.1007/s00268-013-2340-y.
15. Phitayakorn, R., Delaney, C. P., Reynolds, H. L., Champagne, B. J., Heriot, A. G., Neary, P., & Senagore, A. J. (2008). Standardized algorithms for management of anastomotic leaks and related abdominal and pelvic abscesses after colorectal surgery. World Journal of Surgery, 32(6), 1147–1156. doi.org/10.1007/s00268-008-9468-1.
16. Kuehn, F., Janisch, F., Schwandner, F., Alsfasser, G., Schiffmann, L., Gock, M., … Klar, E. (2016). Endoscopic Vacuum Therapy in Colorectal Surgery. Journal of Gastrointestinal Surgery, 20(2), 328–334. doi.org/10.1007/s11605-015-3017-7.
17. Abdalla, S., Cotte, E., Epin, A., Karoui, M., Lefevre, J. H., Berger, A., … Brouquet, A. (2020). Short-term and Long-term Outcome of Endoluminal Vacuum Therapy for Colorectal or Coloanal Anastomotic Leakage: Results of a Nationwide Multicenter Cohort Study From the French GRECCAR Group. Diseases of the Colon and Rectum, 63(3), 371–380. doi.org/10.1097/DCR.0000000000001560.
18. Arezzo, A., Verra, M., Passera, R., Bullano, A., Rapetti, L., & Morino, M. (2015). Long-term efficacy of endoscopic vacuum therapy for the treatment of colorectal anastomotic leaks. Digestive and Liver Disease, 47, 342–345. doi: 10.1016/j.dld.2014.12.003.
19. von Bernstorff, W., Glitsch, A., Schreiber, A., Partecke, L. I., & Heidecke, C. D. (2009). ETVARD (endoscopic transanal vacuum-assisted rectal drainage) leads to complete but delayed closure of extraperitoneal rectal anastomotic leakage cavities following neoadjuvant radiochemotherapy. International Journal of Colorectal Disease, 24(7), 819–825. doi.org/10.1007/s00384-009-0673-7.
20. Verlaan, T., Bartels, S. A., van Berge Henegouwen, M. I., Tanis, P. J., Fockens, P., & Bemelman, W. A. (2011). Early, minimally invasive closure of anastomotic leaks: a new concept. Colorectal Disease, 13 (Suppl. 7), 18–22. doi.org/10.1111/j.1463-1318.2011.02775.x.
21. Glitsch, A., von Bernstorff, W., Seltrecht, U., Partecke, I., Paul, H. & Heidecke, C. D. (2008). Endoscopic transanal vacuum-assisted rectal drainage (ETVARD): an optimized therapy for major leaks from extraperitoneal rectal anastomoses. Endoscopy, 40(3), 192–199. doi.org/10.1055/s-2007-995384.
22. Riss, S., Stift, A., Kienbacher, C., Dauser, B., Haunold, I., Kriwanek, S., … Bergmann, M. (2010). Recurrent abscess after primary successful endo-sponge treatment of anastomotic leakage following rectal surgery. World Journal of Gastroenterology, 16(36), 4570–4574. doi.org/10.3748/wjg.v16.i36.4570.
23. Dioscoridi, L., Forti, E., Pugliese, F., Italia, A., Cintolo, M., Bonato, G., & Mutignani, M. (2020). Response to ‘Endoluminal negative pressure therapy in colorectal anastomotic leaks’. Colorectal Disease, 22(7), 840–841. doi.org/10.1111/codi.15006.
24. Heald, R. J., & Ryall, R. D. (1986). Recurrence and survival after total mesorectal excision for rectal cancer. Lancet (London, England), 1(8496), 1479–1482. doi.org/10.1016/s0140-6736(86)91510-2.
25. McDermott, F. D., Heeney, A., Kelly, M. E., Steele, R. J., Carlson, G. L., & Winter, D. C. (2015). Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. British Journal of Surgery, 102(5), 462–479. doi.org/10.1002/bjs.9697.
26. Pitel, S., Lefèvre, J. H., Tiret, E., Chafai, N., & Parc, Y. (2012). Redo coloanal anastomosis: a retrospective study of 66 patients. Annals of Surgery, 256(5), 806–811. doi.org/10.1097/SLA.0b013e318272de70.
27. Holmgren, K., Kverneng Hultberg, D., Haapamäki, M. M., Matthiessen, P., Rutegård, J., & Rutegård, M. (2017). High stoma prevalence and stoma reversal complications following anterior resection for rectal cancer: a population-based multicentre study. Colorectal Disease, 19(12), 1067–1075. doi.org/10.1111/codi.13771.
28. Vignali, A., & De Nardi, P. (2022). Endoluminal vacuum-assisted therapy to treat rectal anastomotic leakage: A critical analysis. World Journal of Gastroenterology, 28(14), 1394–1404. doi.org/10.3748/wjg.v28.i14.1394.
29. Schiffmann, L., Wedermann, N., Schwandner, F., Gock, M., & Klar, E. (2019). Neoadjuvant radio-chemotherapy prolongs healing of anastomotic leakage after rectal resection treated with endoscopic vacuum therapy. Therapeutic Advances in Gastroenterology, 12, 1756284819877606. doi.org/10.1177/1756284819877606.
30. Talboom, K., Greijdanus, N. G., Ponsioen, C. Y., Tanis, P. J., & Bemelman, W. A. (2022). Endoscopic vacuum-assisted surgical closure (EVASC) of anastomotic defects after low anterior resection for rectal cancer; lessons learned. Surgical Endoscopy, 36(11), 8280–8289. doi.org/10.1007/s00464-022-09274-y.
31. Dindo, D., Demartines, N., & Clavien, P. A. (2004). Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of surgery, 240(2), 205–213. doi.org/10.1097/01.sla.0000133083.54934.ae.
Correspondence:
Svitlana Maliborska
2 Halytska str., Ivano-Frankivsk, 76000
Ivano-Frankivsk National Medical University
E-mail: svetamaliborska13@gmail.com
Адреса для листування:
Маліборська Світлана Віталіївна
76000, Івано-Франківськ, вул. Галицька, 2
Комунальне некомерційне підприємство «Прикарпатський клінічний
онкологічний центр Івано-Франківської обласної ради»
E-mail: svetamaliborska13@gmail.com














Leave a comment