Xanthine oxidase superoxide radicals as a prognostic factors in colorectal cancer metastatic disease
Burlaka Ant.1, Virko S.2, Yefimenko O.1, Zemskov S.3, Ostapenko Y., Lukashenko A.1, Lisnyi I.1, Babak L.1, Burlaka A.4
- 1Nonprofit Organization National Cancer Institute, Kyiv, Ukraine
- 2V.E. Lashkaryov Institute of Semiconductor Physics, Kyiv, Ukraine
- 3Bogomolets National Medical University, Kyiv, Ukraine
- 4R.E. Kavetsky Institute of Experimental Pathology, Oncology and Radiobiology, Kyiv, Ukraine
Summary. Introduction. Obesity is associated with chronic inflammation and colorectal cancer (CRC). In CRC liver metastases and adjacent liver tissue defects are formed in the mitochondrial electron transport chain that causes mitochondrial dysfunction, activation of redox-molecules (superoxide anion (SR), NO) generation sources and matrix metalloproteinases (MMP-2, MMP-9) activity. Radical oxygen species also take a part in the regulation of the xanthine/xanthine oxidase reaction. In human organs xanthine oxidase (XO) is involved in the pathogenesis of various liver diseases. The aim of the study. To determine the state of XO and SR in liver tissue and CRC liver metastases and set the clinical significance of obtained results. Materials and methods. The prospective study on 37 CRC patients who underwent the liver resection due metastatic disease has been performed. The rate of SR generation of neutrophils and in the tissue samples, the rate of XO and the activity of MMP-2, MMP-9 in the tissue samples were measured. Results. The highest level of XO in CRC patients was detected in metastatic tissue (1.47±0.18 a.u.). The level of XO enzyme in the adjacent tissue (AT) and distant tissue (DT) were 0.38±0.10 a.u. (p<0.05) and 0.19±0.008 a.u. (р<0.05) respectively. A correlation was found between the body mass index (BMI) and amount of XO in DT of the liver r2 = 0.64. The total activity of gelatinases in subgroup of CRC patients with BMI ≥25 was found to be significantly higher than in normal body weight patients withing the DT (1.52 times; p<0.001), as well as in the AT (1.28 times; p<0.001). High XO levels (HR (95% confidence interval — CI) = 0.002 (2.63 to 15.05) P = 0.002) and SR generation levels (HR (95% CI) = 0.01 (2.4 to 10.37) P = 0.01) in adjusted liver tissue to metastatic lesions had a negative impact on overall survival rates. Conclusions. XO and superoxide radicals can be a new metastatic disease prognostic factors for CRC patients, the latter requiring further randomized trials.
Received 04.09.2023
Accepted for publication 18.09.2023
DOI: 10.32471/clinicaloncology.2663-466X.51-3.31233
Introduction
Colon cancer — colorectal carcinoma, CRC is the second most common malignant neoplasm of the gastrointestinal tract that lead to more than 500 000 death every year due to the metastatic disease [1]. In our previous works, we established that in CRC patient liver metastases, liver tissue adjacent to metastasis and taken at a distance from metastasis defects are formed in the mitochondrial electron transport chain that causes mitochondrial dysfunction, activation of redox-molecules (SR, NO) generation sources and MMP-2, -9 activity [2].
XO is an enzyme that is a component of the innate immune system and belongs to the molybdenum hydrolase family. The enzyme found consists of two subunits, one molybdenum cofactor (molybdopterin), flavin adenine dinucleotide (FAD), and 8 iron atoms in the iron-sulfur clusters [2Fe-2S] (Fig. 1). XO in the human body exists in two mutually exchangeable forms and catalyzes the last two stages of purine catabolism, using molecular oxygen or NAD+ as electron acceptors. Besides uric acid, a product of XO activity is SR and nitric oxide (NO), which can produce biological effects such as inflammation, endothelial dysfunction, cytotoxicity, mutagenesis, and induction of proliferation. The activity of XO is modulated at the transcriptional and posttranslational levels, and its concentration and activity change significantly in cancer patients [3–5].
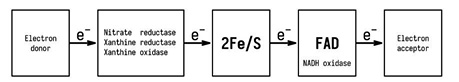
In human organs XO is involved in the pathogenesis of various liver diseases [6]. High levels of XO are found in the liver of rats with different injuries caused by ischemia, hypoxia, hypertension, and cholestasis [7]. Ethanol and γ-irradiation cause increased levels of XO in the liver, are responsible for the exacerbation of tissue damage, increased damage to the affected area, as well as damage to other sites of the body [8]. The main role of XO in the human body is catalyzing transformation from hypoxanthine to xanthine and uric acid by using O2 in the reactions that are associated with the generation of superoxide and reactive oxygen species, which is an important stage of inflammation through activation of the complement system or modulation of endothelial P-selectin expression on the cell membrane, which can play a crucial role in the initiation and progression of CRC [9]. Over the past three decades obesity became an extremely significant public health challenge. Currently more than 33% of the world’s population are overweight or obese [10]. Obesity and related diseases, namely, diabetes mellitus and metabolic syndrome, are associated with chronic inflammation and CRC [11]. Adipose tissue is a dynamic endocrine organ and secrets different proinflammatory cytokines, enzymes, and other factors that activate oxidative stress [12–14]. It is known that MMPs, in particular gelatinases (MMP-2 and -9), play a role in metastatic disease and belong to redox-dependent enzymes, which are regulated by SR and other ROS on levels like synthesis and activation. Due to the fact that ROS take a part in the regulation of MMPs, the xanthine/XO reaction is widely used as a source of exogenous radical oxygen species (ROS) in considering of MMPs.
The aim of the study. To determine the state of XO and SR in liver tissue and CRC liver metastases and set the clinical significance of obtained results.
Materials and Methods
The prospective study has been performed on 37 CRC patients who were treated in the clinic of the National Cancer Institute (Kyiv, Ukraine). All the patients provided informed consent to participate in the research, which was conducted according to the Helsinki Declaration and Ethics Committee of R.E. Kavetsky IEPOR NAS of Ukraine.
Liver tissue sampling protocol. Metastatic and liver tissues sampling was performed intraoperatively (Fig. 2), about ~0.5 cm3 normal liver parenchyma was taken on the ≥5 cm distance (DT) form metastatic lesion (basically in the contralateral liver lobe). Rapidly after the end of the liver transection ~0.5 cm3 adjusted liver tissue (AT) to the metastatic lesion (withing 1 cm of the tumor tissue margin) and ~0.5 cm3 of the metastatic lesion tissue (MT) was taken. Distant liver tissue samples have been taken withing liver transection period or not far than 1 hour after it.
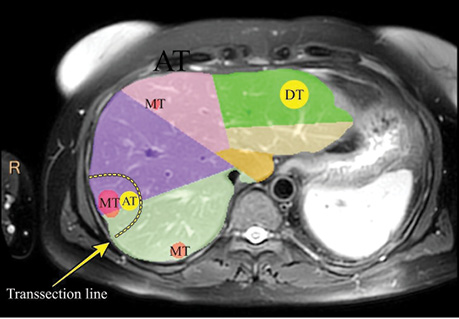
The average age of 37 CRC patients included in this study was 60 y.o. and 59.5% of them were male. The majority of primary tumors has grown into the outermost layers of the colon or rectum (51.4%) or through it (40.5%). Furthermore, 64.8% of the studied patients have had tumor spread into the regional lymph nodes. Metastatic disease spreading is characterized in the Table. In 94.6% liver parenchyma transection was performed under the Intermittent Pringle maneuver with ischemia duration mean 18.5 min. Chemotherapy before liver surgery was performed in 89.2%. It was found that 37.8% of patients had BMI more than 24.9 kg/m2.
| Indicator | Value, n (%) |
| Age, mean (min.–max.), y.o. | 60 (29—74) |
| Male sex | 22 (59.5) |
| Dissemination status of the primary tumor AJCC 6th: | |
| рТ1—pT3 | 22 (59.5) |
| pT4 | 15 (40.5) |
| Status of regional lymph nodes: | |
| pN0 | 13 (35.2) |
| pN1—pN2 | 24 (64.8) |
| Primary tumor: | |
| Right colon | 5 (13.5) |
| Left colon | 32 (86.5) |
| Bilobar metastases | 11 (29.7) |
| Extrahepatic disease | 2 (5.4) |
| Lung metastases | 3 (8.1) |
| Number of the resected lesions, median (min.–max.) | 6 (1–16) |
| Intermittent Pringle maneuver applying | 35 (94.6) |
| Warm ischemia, median (min.–max.), (min.) | 18.5 (0—75) |
| Chemotherapy before liver surgery | 33 (89.2) |
| Chemotherapy courses, median (min.–max.) | 3 (0—8) |
| BMI (kg/m2): | |
| 18.5 to 24.9 | 23 (62.2) |
| 25 to 29.9 | 9 (24.3) |
| ≥30 | 5 (13.5) |
| All patients | 37 (100.0) |
Treatment protocol. Patients received treatment according to approved international standards as well as clinical protocols for the study period from February 2020 to September 2021 [15].
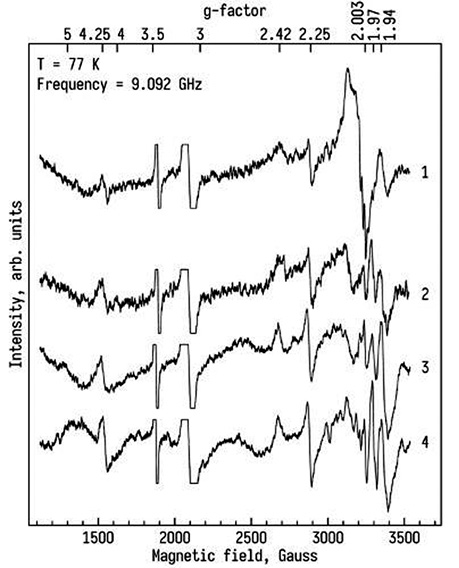
Molecular analysis protocol. The rate of SR generation in mitochondria by NADPH oxidase of neutrophils and tissue samples was determined using a method of EPR and the technology of Spin Traps [16, 17]. The rate of XO in the tissues was determined by the EPR method, and the intensity of the EPR signal, with values of g=1.97, on the EPR spectra corresponds to the level of active XO. The state of the intracellular matrix of the liver was assessed by the activity of matrix metalloproteinases (MMP-2 and -9) and was determined by gelatine zymography in the polyacrylamide gel. The gelatinase activity was expressed in arbitrary units (arb.units) referred to the activity of 1 µg of enzyme per 1 g of the tissue in blood serum and in urine — 1 µg of enzyme per 1 g of the creatinine [18].
Statistical analysis. Descriptive data are presented with numbers means ± standard error of mean (SD) and percentages. To identify predictors of survival multivariable analyses was done using the Cox proportional hazards model. The data were statistically processed using the R statistical package and Prism 9.0. The difference was considered statistically significant at р<0.05.
Results and Discussion
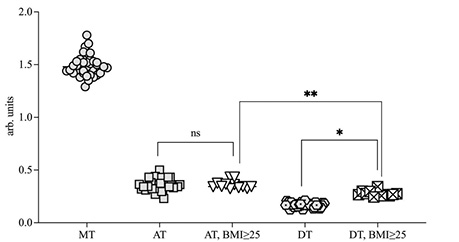
The level of XO enzyme in the AT and DT were 0.38±0.10 a.u. (p<0.05) and 0.19±0.008 a.u. (р<0.05) respectively. And the highest level of XO in CRC patients were detected in metastatic tissue (1.47±0.18 a.u.). Whereas in patients with BMI ≥25 kg/m2 the XO level in the DT liver increased and was found at 0.27±0.12 a.u. (p<0.05). (Fig. 3, 4). A correlation was found between the BMI and amount of XO in DT of the liver r2 = 0.64. Furthermore, in the AT, the XO level was also statistically higher compared to the one in DT (Fig. 4).
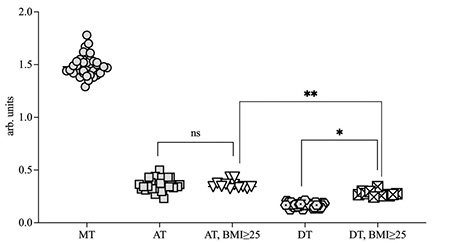
Accumulation of adipose tissue in the body leads to increased levels of XO in the liver, which may contribute to the activation of lipolysis and increase the level of free fatty acids. And increasing levels of XO leads levels-up the intracellular concentration of uric acid, which reacts with ROS and NO and can promote oxidation and proinflammation processes [14].
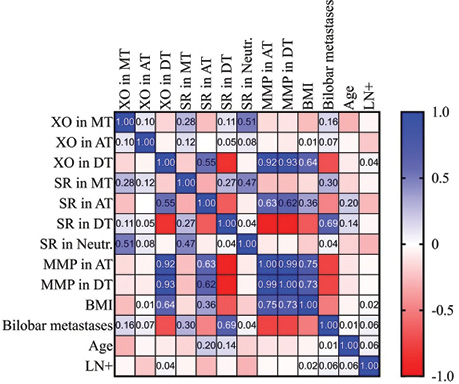
The rate of SR generation in MT was estimated at 1.21±0.09 nM/g tissue•min. And the AT and the DT tissue samples showed 1.59±0.03 nM/g tissue•min and 0.86±0.06 nM/g tissue•min respectively. In the subgroup of patients with BMI ≥25 the SR rate generation was 1.63•0.02 nM/g tissue•min and 0.8•0.007 nM/g tissue•min in AT and DT samples respectively. And strong positive correlation (r = 0.55) between SR speed generation and XO levels in AT was found (Fig. 5).
The matrix metalloproteinases (MMPs) are responsible for tissue remodeling and degradation of the extracellular matrix (PM) proteins both in normal physiological changes in the body and many pathological processes [19–21]. Determined that activity of the total MMP-2 and MMP-9 activity of in the normal and overweight AT samples, exceeded that in the DT samples (by 1.5 and 1.8 times, respectively; p<0.001). The strong positive correlation was registered between the levels XO measured in DT and MMP levels in AT and DT samples r = 0.93 and BMI r = 0.64 (Fig. 6).
The results obtained are confirmed by the works of other authors, whose data demonstrate the dependence between the activity of the studied enzymes, caused, presumably, by the regulatory properties of XO over MMP. Nowadays there are available data about the regulation of MMPs by XO, part of it was obtained in studies of cardiovascular disease, in the progression of which MMPs play an important role — change the elasticity of the vessel wall, modulate various cellular and signaling pathways in atherosclerosis, which are responsible for the formation and removal of atherosclerotic plaques and thrombus [22].
Progression of the tumor to malignancy with visceral obesity is promoted by oxidative stress, an altered adipokines balance, which leads to increased production of insulin and sex hormones which promotes proliferation of cells. Chronic inflammation corresponds to angiogenesis and is the consequence of both increased production of cytokines by dysfunctional adipose tissue, and proinflammatory ROS action, which is formed as a result of XO activity.
The increased XO activity in patients with CRC indicates the role of this parameter as a marker of disease evolution, that it can influence the progression of the disease. A strong correlative relationship between XO and superoxide generating activity of neutrophils indicates a mutual influence on the progression of the tumor (r2 = 0.51). The results of Cox regression in this study revealed that the high XO levels (HR (95% CI) = 0.002 (2.63 to 15.05) P = 0.002) and SR generation levels (HR (95% CI) = 0.01 (2.4 to 10.37) P = 0.01) in adjusted liver tissue to metastatic lesions had a negative impact on overall OS.
High levels of XO and SR in the tumor may be due to increased substrate formation for XO (hypoxanthine→xanthine→uric acid), which increases during the tumor growth. We can already assume that XO levels in these tissues can be used as a biomarker of the CRC metastatic disease progression and further research are needed.
CONCLUSIONS
The highest level of XO was found in metastatic tissue samples. The XO and superoxide radicals high levels in adjusted liver tissue to metastatic lesions had correlations with poor overall survival rates. Obesity correlates with increased generation of superoxide radicals and XO activity, which promotes metastasis. XО and superoxide radicals can be a new metastatic disease prognostic factors for CRC patients, the latter requiring further randomized trials.
Acknowledgements. Not applicable.
Funding. Ministry of Heath of Ukraine.
Availability of data and materials. The data generated in the present study may be requested from the corresponding author.
Authors’ contributions. Anatoliy Burlaka is the main idea creator and molecular researcher. All other co-authors have contributed equally in creating of this manuscript.
Ethical approval. Ethical approval for this study was obtained from the NCI Ethics Service committee (Project ID 76 of 15.01.2015).
Patient consent for publication. Not applicable.
Competing interests. The authors declare that they have no competing interests.
References
1. Patel, S. G., Karlitz, J. J., Yen, T., Lieu, C. H., & Boland, C. R. (2022). The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterology & Hepatology, 7(3), 262–274. doi: 10.1016/S2468-1253(21)00426-X.
2. Burlaka, A. P., Ganusevich, I. I., Lukin, S. M., Vovk, A. V., & Virko, S. V. (2017). Superoxide- and NO-dependent mechanisms of formation of metastatic microenvironment distant sites of metastasis in patients with colorectal cancer. Oncology, 19(1), 64–70.
3. Bortolotti, M., Polito, L., Battelli, M. G., & Bolognesi, A. (2021). Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biology, 41, 101882. doi: 10.1016/j.redox.2021.101882.
4. Veljković, A., Hadži-Dokić, J., Sokolović, D., Bašić, D., Veličković-Janković, L., Stojanović, M., … Kocić, G. (2020). Xanthine Oxidase/Dehydrogenase Activity as a Source of Oxidative Stress in Prostate Cancer Tissue. Diagnostics (Basel), 10(9), 668. doi: 10.3390/diagnostics10090668.
5. Engerson, T. D., McKelvey, T. G., Rhyne, D. B., Boggio, E. B., Snyder, S. J., & Jones, H. P. (1987). Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. Journal of Clinical Investigation, 79(6), 1564–1570. doi: 10.1172/JCI112990.
6. Stirpe, F., Ravaioli, M., Battelli, M. G., Musiani, S., & Grazi, G. L. (2002). Xanthine oxidoreductase activity in human liver disease. American Journal of Gastroenterology, 97(8), 2079–2085. doi: 10.1111/j.1572-0241.2002.05925.x.
7. Furuhashi, M. (2020). New insights into purine metabolism in metabolic diseases: role of xanthine oxidoreductase activity. American Journal of Physiology-Endocrinology and Metabolism, 319(5), E827–E834. doi: 10.1152/ajpendo.00378.2020.
8. Vorbach, C., Harrison, R., & Capecchi, M. R. (2003). Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends in Immunology, 24(9), 512–517. doi: 10.1016/s1471-4906(03)00237-0.
9. Gill, J. G., Piskounova, E., & Morrison, S. J. (2016). Cancer, Oxidative Stress, and Metastasis. Cold Spring Harbor Symposia on Quantitative Biology, 81, 163–175. doi: 10.1101/sqb.2016.81.030791.
10. Brethauer, S. A. (2014). ASMBS Position Statements. The ASMBS Textbook of Bariatric Surgery, 149–155. doi.org/10.1007/978-1-4939-1206-3_13.
11. O’Sullivan, D. E., Sutherland, R. L., Town, S., Chow, K., Fan, J., Forbes, N., … Brenner, D. R. (2021). Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clinical Gastroenterology and Hepatology, 20(6), 1229–1240.e5. doi: 10.1016/j.cgh.2021.01.037.
12. Hashimoto, T., Fukunari, A., Yamada, I., Yanaka, N., Chen, D., & Kato, N. (2005). Y-700, a novel inhibitor of xanthine oxidase, suppresses the development of colon aberrant crypt foci and cell proliferation in 1,2-dimethylhydrazine-treated mice. Bioscience, Biotechnology, and Biochemistry, 69(1), 209–211. doi: 10.1271/bbb.69.209.
13. Griguer, C. E., Oliva, C. R., Kelley, E. E., Giles, G. I., Lancaster, J. R. Jr., & Gillespie, G. Y. (2006). Xanthine oxidase-dependent regulation of hypoxia-inducible factor in cancer cells. Cancer Research, 66(4), 2257–2263. doi: 10.1158/0008-5472.CAN-05-3364.
14. Battelli, M. G., Bortolotti, M., Polito, L., & Bolognesi, A. (2019). Metabolic syndrome and cancer risk: The role of xanthine oxidoreductase. Redox Biology, 21, 101070. doi: 10.1016/j.redox.2018.101070.
15. Benson, A. B., Venook, A. P., Al-Hawary, M. M., Arain, M. A., Chen, Y. J., Ciombor, K. K., … Gurski, L. A. (2021). Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network, 19(3), 329–359. doi: 10.6004/jnccn.2021.0012.
16. Burlaka, A. P., Ganusevich, I. I., Virko, S. V., Burlaka, A. A., & Kolesnik, O. O. (2019). Tumor-associated redox state in metastatic colorectal cancer. Experimental Oncology, 41(2), 148–152. doi: 10.32471/exp-oncology.2312-8852.vol-41-no-2.13128.
17. Burlaka, A. P., Ganusevich, I. I., Gafurov, M. R., Lukin, S. M., & Sidorik, E. P. (2016). Stomach Cancer: Interconnection between the Redox State, Activity of MMP-2, MMP-9 and Stage of Tumor Growth. Cancer Microenviron, 9(1), 27–32. doi: 10.1007/s12307-016-0182-5.
18. Burlaka, A. P., Ganusevich, I. I., Lozovska, Y. V., Lukyanova, N. Y., & Chekhun, V. F. (2015). Redox-regulation of gelatinases during growth of cisplatin-sensitive and resistant Guerin carcinoma. Experimental Oncology, 37(1), 36–39.
19. Quintero-Fabián, S., Arreola, R., Becerril-Villanueva, E., Torres-Romero, J. C., Arana-Argáez, V., Lara-Riegos, J., … Alvarez-Sánchez, M. E. (2019). Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Frontiers in Oncology, 9, 1370. doi: 10.3389/fonc.2019.01370.
20. Mori, K., Uchida, T., Yoshie, T., Mizote, Y., Ishikawa, F., Katsuyama, M., & Shibanuma, M. (2019). A mitochondrial ROS pathway controls matrix metalloproteinase 9 levels and invasive properties in RAS-activated cancer cells. FEBS Journal, 286(3), 459–478. doi: 10.1111/febs.14671.
21. Liu, W., Rosenberg, G. A., Shi, H., Furuichi, T., Timmins, G. S., Cunningham, L. A., & Liu, K. J. (2004). Xanthine oxidase activates pro-matrix metalloproteinase-2 in cultured rat vascular smooth muscle cells through non-free radical mechanisms. Archives of Biochemistry and Biophysics, 426(1), 11–17. doi: 10.1016/j.abb.2004.03.029.
22. Olejarz, W., Łacheta, D., & Kubiak-Tomaszewska, G. (2020). Matrix Metalloproteinases as Biomarkers of Atherosclerotic Plaque Instability. International Journal of Molecular Sciences, 21(11), 3946. doi: 10.3390/ijms21113946.
Correspondence:
Anton Burlaka
33/43 Yulia Zdanovska str., Kyiv, 03022
Nonprofit Organization National Cancer Institute
E-mail: nir.burlaka@gmail.com
Адреса для листування:
Бурлака Антон Анатолійович
03022, Київ, вул. Здановської Юлії, 33/43
Державне некомерційне підприємство «Національний інститут раку»
E-mail: nir.burlaka@gmail.com














Leave a comment